Manufacturing of lithium-ion cells and batteries began in the 1990s with applications in portable electronic equipment from cameras to camcorders. This battery chemistry was then introduced into the laptop market and the applications using these grew significantly, increasing the demand. Today, batteries are used in a myriad of applications and range from low energy powering portable electronics with tens of watt-hours (Wh) to higher energy in kilowatt-hours (kWh) powering electric vehicles and further in megawatt-hours (MWh) and gigawatt-hours (GWh) sizes in grid energy storage applications. Due to the increased demand, the number of manufacturers in the area of lithium-ion cell and battery manufacturing has also increased significantly.
Components that go into manufacturing a lithium-ion cell such as the cathode, anode, separator, electrolyte, to name a few, are constantly undergoing optimization with discoveries that give rise to higher energy and power densities. Hence, the manufacturing process is not a straightforward mundane process. The nature of lithium-ion cell and battery manufacturing requires stringent process control since low quality, the presence of defects, and the lack of relevant design controls can lead to catastrophic failures. With the mushrooming of cell and battery manufacturers in this industry, it has been noted that those manufactured with a lack of quality and configuration control are at a higher risk for catastrophic failures such as fire, smoke, and thermal runaway in-field use. Thermal runaway occurs when the heat produced in a cell or battery is higher than the heat dissipated from it resulting in a rapid temperature rise and typically accompanied by fire and/or smoke.
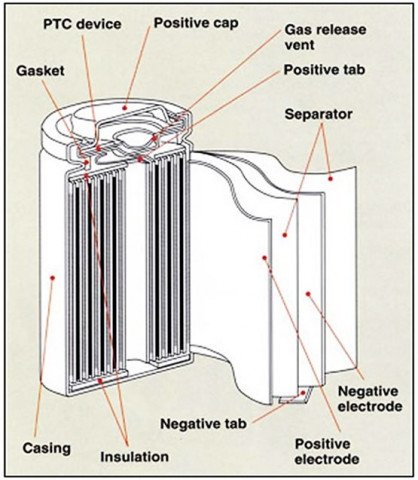
To start with, let us look at the four main components that go into making a cell (Figure 1). These are the cathode (positive electrode), the anode (negative electrode), the separator (provides physical separation between the cathode and anode), and the electrolyte (provides a medium for the conduction and passage of ions). The example provided in Figure 1 is that of a lithium-ion 18650 cell that also has other components that go into the cell.
When designing cells and batteries, thermodynamic and kinetic processes internal to the cell and battery can affect the voltage, current, and temperature of the cells. This is attributed to the three types of polarizations associated with any cell or battery (Figure 2). The three are, namely, ohmic polarization, concentration polarization, and activation polarization. The ohmic polarization is the major contributor to the losses observed in voltage and is caused due to many factors such as contact resistance (welds, solders, adherence of active material to current collector), ionic resistance of the electrolyte (viscosity), interfacial resistance between electrode and electrolyte, nature of the electrodes (porosity and packing density), among others.
The concentration polarization is dictated by the ability of the ions to move from one electrode to the other and the availability of active materials on the surface of the electrodes for the electrochemical process to occur. The activation polarization drives the electrochemical reaction and is determined by the overlap of the energy wells for the electrodes used. The activation polarization is determined by the choice of cathode and anode materials and is not influenced by the quality of the manufacturing process whereas the other two polarizations play a major role in the performance, and to a certain extent, in the safety of the cell and eventually the battery.
To reduce the adverse effect of these polarizations, one should, as a minimum, take into account the following:
The ionic conductivity of the electrolyte should be maximized to reduce the ohmic polarization and select electrolyte salts and solvents that are chemically and electrochemically stable with the electrodes.
The electrode reaction rates should be confirmed to be sufficiently high to minimize activation polarization.
Confirm that the ionic conductivity of the electrolyte is sufficiently high to reduce concentration polarization and that the main reaction products remain at the electrode surface for rechargeable systems.
Choice of current collectors should be such that they are compatible with the electrodes and the electrolyte, and have low contact resistance.
Ensure that the electronic conductivity of these is maximized.
These steps will lead to a minimization of losses due to the three different polarizations eventually reducing the losses in operating voltage and increasing the performance of the cells and batteries.
Let us now focus on lithium-ion cells and batteries and the importance of manufacturing in maximizing performance and safety. When lithium-ion cells became commercially available in the late 1990s, the first available design was the cylindrical metal can 18650 model (Figure 1) wherein the diameter of the cell was 18 mm and the length (height) of the cell was 65 mm. In the next decade, other cell sizes and formats became available. Two of the common formats were the prismatic metal can (Figure 3a) and prismatic pouch (Figure 3b) types. Within the prismatic cell formats, different methods of electrode assembly have been used of which the elliptical wound (Figure 3c), stacked (Figure 3d), and Z- fold electrodes (Figure 3e) is the most common.
The following section of the article discusses the process of cell manufacturing and the need for cleanliness, dry rooms, and quality control. Figure 4 describes the main steps involved in manufacturing the electrodes.
The information under each box that carries the various stages of the manufacturing process, guides what needs to be taken care of to have an astringent and quality-controlled process for the manufacturing of the electrodes. Some factors that require reiteration are the confirmation that cross-contamination of cathode and anode active materials should be avoided, and that during the processes where metal particles could be incorporated as with the mixing and slitting processes. The use of magnets in appropriate locations prevents the introduction of undesired metal particles or filings into the cell components that can later result in a catastrophic failure.
It is also imperative to check the uniformity of the electrode thickness and an inspection of the electrodes, using random samples from the electrode roll, to confirm the uniformity of the electrodes, the lack of pinholes, the lack of lumps and other impurities, and their adherence to the current collector. The requirements for a cleanroom should also be followed as required for the various steps. Utmost care must be taken to maintain a clean working environment and maintain strict quality control.
Figures 5 and 6 provide a detailed account of the steps involved in manufacturing the cells. The factors that need to be given attention during the manufacturing of each of the electrodes as well as the incorporation of the electrodes with the separator into the cell container are detailed in Figure 5. The noteworthy ones are to confirm that:
there is no introduction of metal or foreign particles,
that there are no defects or deformations introduced due to lack of care exercised during the electrode roll insertion process,
the lack of faulty or improper welds, prevention of weld splatters,
the use of proper heat-sealing techniques for pouch format cell sealing, and
the addition of accurate volume and high purity electrolyte in the cells.
Once the electrode roll or stack is placed inside the cell container and the electrolyte has been added, it is necessary to complete the formation cycle wherein the charging of the cell takes place in addition to the removal of bubbles and air gaps within the cell. Once this is completed, the cell is sealed in a relevant manner for the metal can and the pouch format cells. After the cell is sealed, several steps are performed as described in Figure 6 where the cells are X-rayed to confirm that the electrode roll/stack is uniformly aligned and that there is no impingement of current collector tabs on the separator or electrode causing compression or the path to create a short circuit between the cathode and anode. Cell resizing or height adjustment performed as the last step during cell manufacturing can cause issues (Figure 7) and it should be performed before X-rays and washing of the cell. Washing the cell before height adjustment pushes electrolyte that is squeezed out at the crimp seal. The electrolyte along with moisture in the atmosphere causes corrosion of the cell can (steel) in the crimp area.
Storage aging is a very significant step in the cell manufacturing process, since the period of rest provides significant data on early cell failures due to inadvertent defect introduction or poor cell build.
The production quality of cells can be determined in the following manner. The data from the entire cell lot should be reviewed for dimensions, mass, open-circuit voltage (OCV), capacity, and internal resistance / ac impedance. A statistical analysis of the data from the measurements mentioned from each lot should be performed and if there is more than 3 percent of the cells that lie outside the 6-sigma range, the lot should not be delivered for use in the build of batteries. In addition to this, if there is more than a 10 percent failure rate after the cell formation cycle for each lot, then the lot shall not be used or delivered for the manufacturing of batteries.
So, why is having a high-quality cell and battery manufacturing process critical to safety? An internal short circuit is a credible hazard associated with lithium-ion cells that can result in a catastrophic failure of cells and batteries in the field. Internal short circuits are caused by two means: (i) Due to defects created during the manufacturing process that were not detected or screened out and that manifests themselves in the field while in use or storage; and (ii) Due to bad design or misuse where overcharge (overvoltage), over-discharge, extreme high and low temperatures and external short circuits can induce internal shorts to be developed in the field. High-quality cell and battery manufacturing processes reduce the risk of an internal short manifesting itself during the manufacturing process as well as in the field.
When one does not have the ability to audit the cell manufacturing facility, certain steps can be taken to confirm the quality of the calls received. The analysis and criteria suggested above to determine the production quality of cells is an important step. Apart from that, destructive physical analysis (DPA) of a random set of cell samples from the lot can help determine the quality of the lot received. Factors that need attention are the following:
The length of the anode electrode should be more than the cathode electrode and the separator should be longer than both electrodes; electrodes and separator should be aligned with no tunneling.
All current collectors that lead from the electrode stack to the terminals should be directed in a way to avoid contact with the electrodes and have strain relief to prevent breakage;
Separators and electrodes should not have any creases, folds, etc., that can become nucleation areas for lithium dendrite formation or salt deposits during the field use (charge and discharge).
Physical abnormalities, such as bumps, tears, pinholes, foreign object debris, native object debris, non-uniform electrode thickness, changes in appearance, delamination, and electrode dry out areas should not be present; welds should be uniform with no protrusions or weld splatters.
All metal surfaces that include current collector tabs, exposed metal current collector at the end or beginning of the wind, etc., should be covered with insulating material like Kapton tape or equivalent.
Plastic or other non-conductive washer type insulators should be used wherever isolation between active materials is required; for instance, between the electrode roll and the bottom of a 18650 cell can; between the top of the electrode roll and the header of the cell, and between the top of the electrode and the terminal leading to the header, etc.
An extra layer of the separator that isolates the electrode roll from the cell can, even those with positive or negative potential.
Insulation of cell cans that are deemed neutral (floating potential) should have isolation checks performed.
A brief description of best practices for high-quality battery build includes the following:
All lithium-ion batteries should have a battery management system (BMS) that provides monitoring of cell (cell bank) voltages, series string current, and temperature with controls for overvoltage, under-voltage, short circuit currents, and unsafe temperatures.
Cell balancing is imperative and the presence of state of health monitoring should be provided for relevant applications that require long-term performance.
The components that are used in the batteries such as wire gauge, MOSFET (metal-oxide-semiconductor field-effect transistor) switches, fuses, diodes, and other electronic components should be rated for the application.
The software used for performance and safety should be verified to work appropriately.
All cells that go into a battery should be from the same lot that has the same manufacturing date code.
In the case of high-energy batteries such as those used in electric vehicles and stationary grid storage applications, the heat dissipation paths and thermal gradient within the battery should be well understood to obtain a design with the least thermal gradient possible (preferably within 3 °C).
Finally, the battery should be verified by test to provide the relevant performance as well as a confirmation that the safety controls work at the various levels as required.
Cells and batteries should be certified using the relevant performance and safety standards available. Some of the standards that can be used to certify cells are UL 1642, UL 62133-2 / IEC 62133-2. Some of the standards that are relevant for consumer battery certifications are UL 2054, UL 2056, UL 8139, UL 2272, UL 2849, and UL 3030.
Stringent quality manufacturing processes for cells and batteries can reduce field failures due to latent defects and improve their safety. The use of high-quality cells and batteries provides a safer transportation and usage environment.
Author: Judith Jeevarajan, Ph.D., Research Director, Underwriters Laboratories
(The author would like to thank her team members for their valuable comments. More information regarding lithium-ion battery safety studies can be obtained by contacting [email protected].)
Read More





 Policy & Regulatory Advocacy
Policy & Regulatory Advocacy