Flow battery systems have been under development since the 1970s and have evolved tremendously since then. Many variations of this technology are commercially available and many more are in the research stage.But what is a flow battery and how is it different from any other battery? To answer this, we need to first understand its construction and how it works.
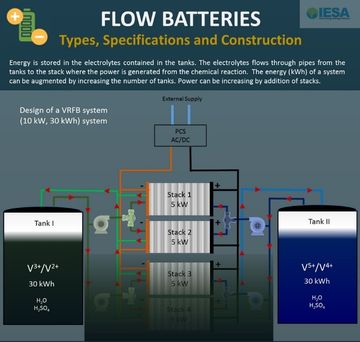
All the energy in a flow battery is stored in its two electrolytes. These electrolytes are stored separately in two or more tanks. Whenever energy is required, the two electrolytes are flown through a network of pipes and pumps to reach the stack. The stack is the heart of the system where the reaction takes place and electricity is generated. In this sense a flow battery is much like a car engine, where the petrol or diesel is analogous to the electrolytes and the engine to the stack.
One of the unique things about a flow battery is that the power and energy are decoupled. This is because the total energy (kWh) stored depends on the volume of the electrolytes. In the example shown, the tanks have enough electrolyte to be able to provide 30 kWh of energy. If we double the number of tanks we would have 60 kWh of energy. Similarly, if we need to increase the power (kW) we can add more stacks. This obviously implies an additional cost which needs to be financially justified. But it is the unique design of the flow battery which permits this flexibility in fine tuning the specifications based on the application requirements.
The variations
Like we discussed in the article about Li-ion batteries in the previous ETN issue, the term flow batteries also refers to a family of technologies. All of these share many similarities in their construction but differ in their chemistry. Currently, the most mature technology in this family is the Vanadium Redox Flow Battery (VRB). In this chemistry, the active element in both the electrolytes is Vanadium (V). That is why it is denoted by the term V/V in the table. Another variation is the Zinc-Iron (Zn/Fe) flow battery which has the active element Zn in one electrolytes and Fe in the other. The low energy density (8-10 Wh/kg) of these batteries is a result of the large weight of their electrolytes. Due to their large weight and volume, flow batteries are only considered suitable for stationary storage applications. The c-rates for most flow batteries are in the range of C/4-C/10, which means that these are adapted for long duration applications. These could include solar backup, microgrids and other applications which require 4-10 hour energy backup. This is where their high cycle life (10000+) makes them really attractive. Typically, a long duration battery will do one cycle a day, in which case one could envision an almost 20 year lifetime for these batteries. VRBs are ideally suited for very large multiple MWh storage systems. Although, the third type of flow battery (Zn/Br) does not exhibit as high a cycle life (3000+), it has an advantage in terms of the compactness (40 – 45 Wh/kg). This is because Zinc-Bromine battery is in fact a hybrid flow battery as only one of its electrolytes (Bromine) is liquid. The other component (Zinc) is a solid. Due to this, its total electrolyte weight is much lesser compared to V/V or Zn/Fe batteries.
What happens in the stack of a VRB?
The stack is where the energy conversion happens. In VRBs, the voltage of a single cell is 1.26 V which is obviously not enough for any real application. A stack consists of several cells stacked in series so as to give a system capable of delivering 48 V or higher as required. In each cell, the two electrolytes flow over the graphite felt electrodes where they react to produce electric current. It is crucial that the two electrolytes do not mix with each other which is why the two chambers of flowing electrolytes are kept separated by a special ion-conducting membrane. Currently, Nafion is the most popularly used membrane type. Each cell is a sandwich of bipolar plates, graphite felt electrodes with the membrane in the centre. There are two major problems related to the stack which often limit the longevity of VRBs. The first one is that of electrolyte leakage and sealing which can lead to decrease of battery capacity over time. The other is that of Vanadium crossover through the membrane. Some of the Vanadium from one electrolyte can pass through the membrane and enter the other electrolyte leading to contamination and also decrease in capacity. Prevention of these two issues is the focus of a significant fraction of the patents on this topic. Membrane material is the largest contributor to the cost of the stack. And according to different estimates, the cost of the stack accounts for 15-25% of the total system cost.
How much Vanadium do we need?
In VRBs, Vanadium is the key active ingredient in the electrolyte which stores the electrical energy. Rest of the liquid electrolyte is composed of water and acid. The Vanadium is obtained from mining and is available in the form of its oxide V2O5. Approximately, 10 tons of V2O5 (same as 5.5 tonnes of Vanadium) is required for producing a 1 MWh battery. This is less than 0.01% of the total annual global production (83,000 tons). Currently, almost 95% of all the Vanadium is used in steel making applications. However, if the VRB industry were to attain an annual production in GWh, the battery industry could account for 15-20% of the total production. It is important to discuss the specifics of Vanadium because it accounts for 91% of the electrolyte cost. The electrolyte cost itself is 38% of the total system cost. Hence, any price fluctuation of this key raw material can have a significant impact on the total system price.
Zinc-Bromine Batteries
n-Br batteries are generally packaged in much smaller modular units of 10 – 150 kWh. These batteries can serve the backup needs for a single household or that of a small community (10-15 houses). It is possible to assemble larger systems of a few MWh which will be suitable for larger communities, office buildings and companies. One of its key advantages is the inexpensiveness of its main constituent raw materials. Zinc is produced on a global scale with an annual production of 13+ million tonnes the annual production of Bromine is 0.35 million tonnes. If an astounding 100 GWh of batteries were being produced annually, it would need only 1.2 % of the total Zn production. This means that the Zn price would remain unaffected. Whereas the Br production would need to be almost doubled to account for the demand generated by Zn/Br batteries. Existing reserves indicate that such demand for Br can be met, but that would necessitate for the mining industry to scale up and grow with the battery industry. Technologically, one simplifying factor in case of Zn/Br batteries is that there is no need for a specialized membrane (such as Nafion) to separate the two electrolytes. This is because when the battery is being charged the active materials automatically separate out as a solid (Zn) and as an insoluble liquid (Br2) from the liquid electrolyte. The zinc deposits on the negative electrode (shown in blue) whereas the bromine collects as a liquid in one of the tanks. However, this also gives rise to one of the main challenges of Zinc dendrite formation, which limits the cycle life. Dendrites are sharp needle like structures which form when the zinc deposits on the negative electrode. These sharps needles can pierce through and damage the membrane as shown in the figure. Ongoing design improvements, majorly on the stack continue to push the boundaries on all performance parameters for Zn-Br batteries.
Recyclability
One of the aspects where flow batteries in general trump other battery technologies is in ease of recycling. This is made possible primarily due to the design of flow batteries which is systemically different from other batteries. In flow batteries, all the active chemicals are stored in the electrolyte tanks. At the end of life, these electrolyte can be drained out of the tanks and sent directly for chemical processing and subsequent re-use in new systems. The remaining components are either mechanical pumps and tubing, electronic components or electrical wiring which can be manually dissembled for appropriate recycling along pre-established routes. In other battery systems such as Lead acid and Li-ion, each cell needs to be crushed and ground into small pieces. From this mixture all the different materials need to be separated out. This is energy and equipment intensive and leads to ample cross-contamination in recycled materials. Currently, flow batteries are at the stage where the storage cost ($/kWh) can benefit immensely from the increase in scale of manufacturing. The demonstration of a long cycle life, scalability to large MWh systems along with the ease of chemical recycling and re-use, surely make them a strong contender for stationary long duration backup applications.





 Policy & Regulatory Advocacy
Policy & Regulatory Advocacy