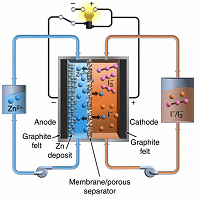
A new type of redox flow battery has an energy density approaching that of lithium-ion batteries used in portable electronic devices and some small electric cars.
A flow battery is an energy storage device based on two chemical components dissolved in liquids separated by a membrane. Unlike a standard deep cycle battery, the electrodes in a flow battery are not surrounded by all the electrolyte in the system, nor are they consumed.
When power is needed, the two liquids are pumped into the central stack and as ions pass through a selective membrane, energy is produced. The battery is ‘recharged’ by reversing the flow.
The general design of a flow battery enables scale and is relatively cheap to produce. Flow batteries are also considered safer than lithium-ion. It’s these qualities that have made flow batteries attractive in relation to renewable energy storage.
However, energy density has been a challenge up until now.
A new zinc-polyiodide redox flow battery developed by Pacific Northwest National Laboratory (PNNL) and described in Nature Communications uses an electrolyte with more than double the energy density of the next best design; opening up possibilities of using flow batteries in applications such as vehicles in the future.
“With improved energy density and inherent fire safety, flow batteries could provide long-duration energy storage for the tight confines of urban settings, where space is at a premium,” said Imre Gyuk, energy storage program manager at the US Department of Energy’s Office of Electricity Delivery and Energy Reliability.
Testing of a small 12 watt-hour zinc-polyiodide redox flow battery demonstrated 167 watt-hours per liter of electrolyte; a big improvement on zinc-bromide (~ 70 watt-hours per liter) and vanadium (~ 15 and 25 watt-hours per liter).
The PNNL team calculated their new battery could theoretically discharge even more – up to 322 watt-hours per liter; which could take it beyond the performance of lithium ion batteries.
Another advantage of the zinc-polyiodide redox flow battery is its ability to deliver high performance in extreme conditions – temperatures as low as -20 degrees Celsius and as high as 50 degrees.
The zinc-polyiodide flow battery isn’t ready for prime time yet. The researchers are still grappling with issues such as managing zinc dendrite formation on the central stack’s negative electrode; which then permeates the membrane, making the battery less efficient.
The team has set its sights on building a larger, 100-watt-hour model of the battery for additional testing.
Funding for the research has been provided by Department of Energy’s Office of Electricity Delivery and Energy Reliability.
(This news story is from Energy Matters)